Born in Salford in 1818, James Joule was the first person to prove that heat is a form of energy. The son of a leading Salford brewer, his experiments combined brilliant scientific thinking with the brewer’s obsession with accurate measurement. And yes, the joule—the standard international unit of energy—is named after him. Think about that next time you’re counting your kilojoule energy intake.
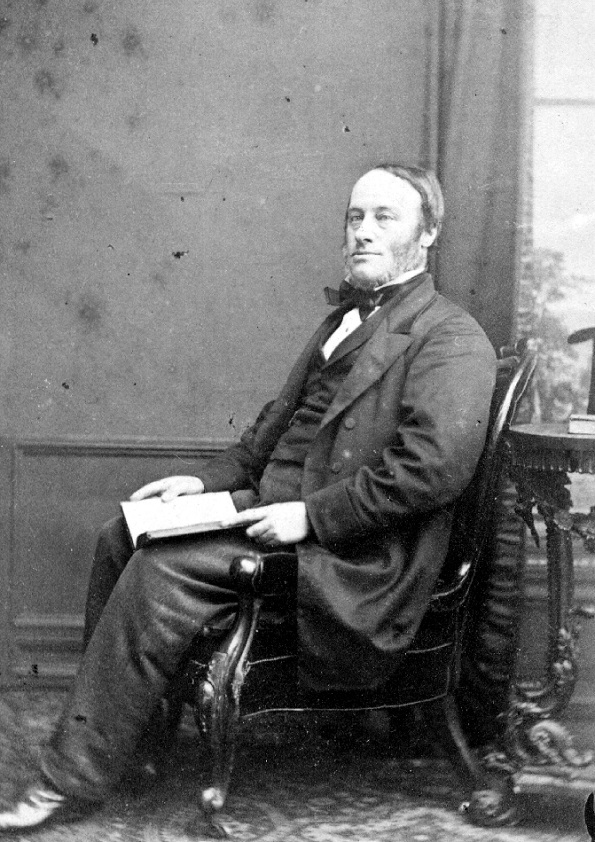
Joule was fascinated by the ways that energy changes from one form into another. He was curious and keen to test out his ideas, and his notes give an insight into the personality of a young man with a passion for experiments. Curious to learn more about how electricity works, Joule records how he gave himself, and even one of his long-suffering servants, electric shocks during his electrical experiments.
Science Museum Group © The Board of Trustees of the Science Museum
Joule’s greatest experiment into the relationship between heat and mechanical energy took place in the stable temperature of his family’s brewery cellar. In his now-famous paddlewheel experiment, the paddlewheel sat in a cylinder of water, with a string wound around its axis. A weight was attached to the other end of the string, and as the weight fell, the string unwound causing the paddlewheel to turn. Joule measured the temperature of the water before and after and found that the water had got slightly warmer, concluding that the work done by the falling weight had been converted into heat through the friction of the paddlewheel stirring the water. Joule’s highly-sensitive thermometer meant that he could measure the tiny temperature increase and from this, he established a numerical relationship between heat and mechanical work.

Science Museum Group © The Board of Trustees of the Science Museum
Joule’s groundbreaking experiments into the relationship between heat and mechanical work were radical in the science world of the 1850s. Before Joule proved his mechanical theory of heat, most scientists subscribed to the caloric theory. They thought that heat was a fluid substance called ‘caloric’ which permeated solids and liquids and flowed from hotter to colder areas. Although completely wrong, many aspects of heat seemed to be explained by the caloric theory. Joule’s work overturned these ideas by proving with his careful experiments that heat was a form of energy. In 1850 he published his research in an explosive article for the Royal Society called On the Mechanical Equivalent of Heat.
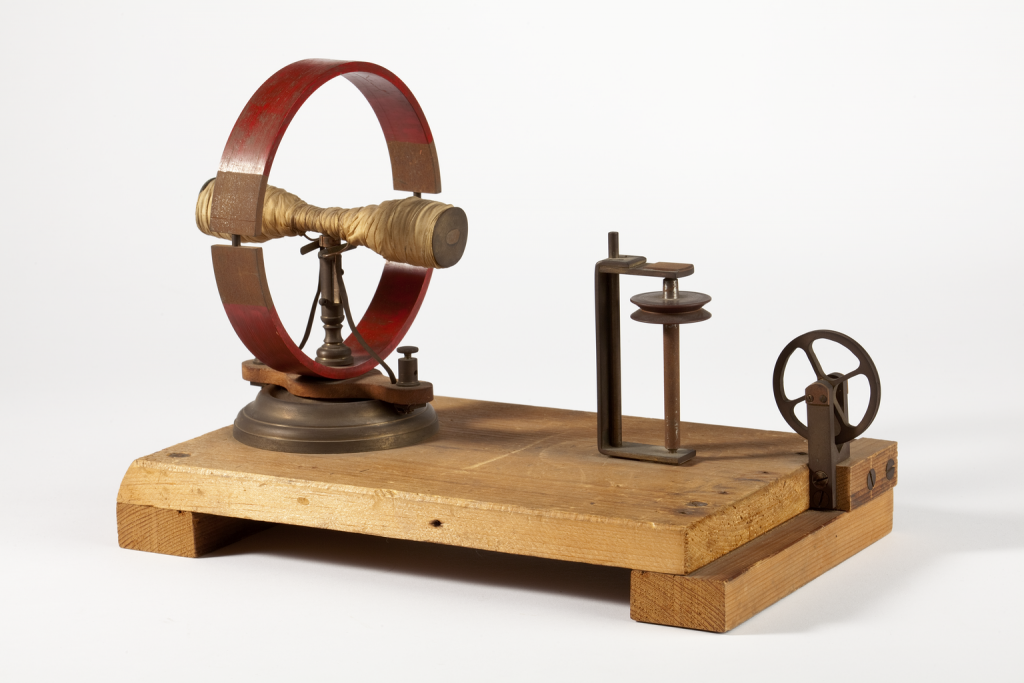
Equipment made and used by Joule in his experiments are highlights of the Science and Industry Museum collection. Accurate measurement of temperature made Joule’s careful heat experiments possible. He was able to measure and record tiny changes of temperature to develop his law of the conservation of energy, which says that energy can be converted between different forms but cannot be destroyed.
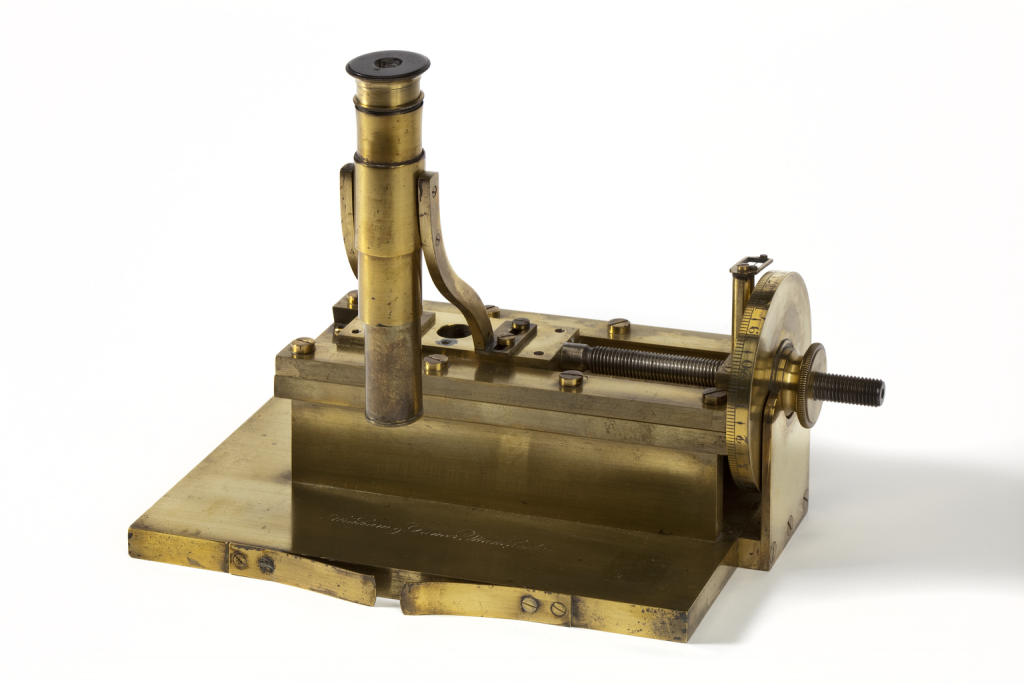
Joule couldn’t have made such fine measurements without the help of skilled instrument makers. This ‘travelling’ microscope was made for Joule by Abraham and Dancer in 1843. He used it to calibrate his thermometers. Some scientists simply refused to believe the accuracy of measurements Joule achieved in his experiments. His repeated experiments and precise results finally won over his critics and his ideas became accepted as the standard theory of heat.
Science Museum Group © The Board of Trustees of the Science Museum
To celebrate 200 years since James Prescott Joule’s birth this December, we’ve created a display about him in the Reading Room at Central Library. The display showcases some of the original equipment used by Joule in his ground-breaking experiments into heat, energy and electricity, and is at the library until January.
Our display also looks at how Joule’s work led to huge improvements in the efficiency and safety of steam engines, the primary motive power of the day. Harnessing the power of steam was a dangerous business and before heat was well understood, tragic boiler explosions happened quite frequently.
Joule’s contribution to a proper understanding of the relationship between heat and energy helped to make engines safer, and also much more efficient. By creating an exact value for converting heat into work, Joule gave engineers a practical way to calculate the efficiency of their steam engines. Precise mathematics could now be used to create new, highly advanced machines.
It is strange to think that the idea that heat is a form of energy, so fundamental to our understanding of the physical world, was developed by Joule here in Manchester 170 years ago. Joule’s theory on the nature of heat was so controversial at the time that at first he could not find a scientific journal to publish it. News of his famous discovery first appeared in a local Manchester newspaper, the Manchester Courier. Later when his theories had been tried, tested and accepted, he became celebrated as one of the world’s most famous scientists.

(CC BY-SA 3.0)
An interesting article. It does beg the question: what was special about the construction of his thermometers such that they could be so accurate? Are there any still about? One would imagine that with his theories being investigated and accepted many people would need one.